Our Health Library information does not replace the advice of a doctor. Please be advised that this information is made available to assist our patients to learn more about their health. Our providers may not see and/or treat all topics found herein.
Hereditary Diffuse Gastric Cancer (PDQ®): Genetics - Health Professional Information [NCI]
This information is produced and provided by the National Cancer Institute (NCI). The information in this topic may have changed since it was written. For the most current information, contact the National Cancer Institute via the Internet web site at http://cancer.gov or call 1-800-4-CANCER.
Introduction
Hereditary diffuse gastric cancer (HDGC) is an autosomal dominant hereditary cancer syndrome that increases an individual's risk to develop diffuse gastric cancer and lobular breast cancer. HDGC is defined by the presence of germline pathogenic variants in the CDH1 gene, which codes for the cell–cell adhesion junction protein, E-cadherin. In patients with CDH1 pathogenic variants, early stage gastric cancer is characterized by intramucosal foci of signet ring cell carcinoma while advanced gastric cancer presents as poorly cohesive diffuse type carcinoma with very few typical signet ring cells.[1] In CDH1 pathogenic variant carriers, lifetime risk of diffuse gastric cancer (to age 80 y) ranges from 37% to 70% in men and 25% to 83% in women.[2,3,4,5] The average age of gastric cancer diagnosis can range from 38 to 80 years, with the earliest reported diagnosis occurring at age 14 years.[2,3,4,6,7] The lifetime risk for lobular breast cancer (to age 80 y) is 39% to 55% in women who carry a CDH1 pathogenic variant. The average age of lobular breast cancer diagnosis ranges from 46 to 50 years.[2,4,8] Recent gastric and lobular breast cancer risk estimates are even lower than these numbers [9] because clinical criteria for genetic testing are becoming less stringent. This phenomenon has also been seen with other hereditary cancer syndromes, like hereditary breast and ovarian cancer and Lynch syndrome.
Pathogenic variants in a second gene, CTNNA1, are found in a small number of families with HDGC. This gene codes for an adhesion junction protein, catenin alpha-1.[10,11] The age of gastric cancer onset in confirmed CTNNA1 pathogenic variant carriers ranges from 22 to 72 years. The first study to provide CTNNA1 penetrance estimates reported up to a 57% lifetime risk of developing gastric cancer in families from European hereditary cancer registries.[12] It is unclear if CTNNA1 pathogenic variants increase breast cancer risk.[13] Given the limited knowledge about the prevalence and penetrance of CTNNA1 pathogenic variants, many commercial testing laboratories do not offer clinical genetic testing for this gene.
References:
- van der Post RS, Gullo I, Oliveira C, et al.: Histopathological, Molecular, and Genetic Profile of Hereditary Diffuse Gastric Cancer: Current Knowledge and Challenges for the Future. Adv Exp Med Biol 908: 371-91, 2016.
- Pharoah PD, Guilford P, Caldas C, et al.: Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121 (6): 1348-53, 2001.
- Roberts ME, Ranola JMO, Marshall ML, et al.: Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol 5 (9): 1325-1331, 2019.
- Xicola RM, Li S, Rodriguez N, et al.: Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet 56 (12): 838-843, 2019.
- Hansford S, Kaurah P, Li-Chang H, et al.: Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 1 (1): 23-32, 2015.
- Delgado E, León-Ponte M, Villahermosa ML, et al.: Analysis of HIV type 1 protease and reverse transcriptase sequences from Venezuela for drug resistance-associated mutations and subtype classification: a UNAIDS study. AIDS Res Hum Retroviruses 17 (8): 753-8, 2001.
- Caldas C, Carneiro F, Lynch HT, et al.: Familial gastric cancer: overview and guidelines for management. J Med Genet 36 (12): 873-80, 1999.
- Corso G, Intra M, Trentin C, et al.: CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer 15 (2): 215-9, 2016.
- van der Post RS, Vogelaar IP, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 52 (6): 361-74, 2015.
- Majewski IJ, Kluijt I, Cats A, et al.: An α-E-catenin (CTNNA1) mutation in hereditary diffuse gastric cancer. J Pathol 229 (4): 621-9, 2013.
- Benusiglio PR, Colas C, Guillerm E, et al.: Clinical implications of CTNNA1 germline mutations in asymptomatic carriers. Gastric Cancer 22 (4): 899-903, 2019.
- Coudert M, Drouet Y, Delhomelle H, et al.: First estimates of diffuse gastric cancer risks for carriers of CTNNA1 germline pathogenic variants. J Med Genet 59 (12): 1189-1195, 2022.
- Clark DF, Michalski ST, Tondon R, et al.: Loss-of-function variants in CTNNA1 detected on multigene panel testing in individuals with gastric or breast cancer. Genet Med 22 (5): 840-846, 2020.
Genetic Testing Criteria for Hereditary Diffuse Gastric Cancer (HDGC)
The International Gastric Cancer Linkage Consortium's (IGCLC) 2020 HDGC genetic testing guidelines are summarized below.[1]CDH1 genetic testing can be conducted when an individual meets the IGCLC's HDGC genetic testing guidelines and when the following items from the personal history or family history criteria below are met. It is recommended that cancer diagnoses and histologies in the proband and/or family members are validated with appropriate medical records. Furthermore, when two or more different cancer types are involved, at least one must be diffuse gastric cancer with signet ring cell carcinoma or lobular breast cancer. Intestinal-type gastric cancers and non-lobular breast cancers cannot be used to meet HDGC genetic testing criteria (even if the histologies of these tumors have been validated), because these cancers are not associated with HDGC. For more information, see the Genetic Evaluation in Individuals With Personal and/or Family Histories of Gastric Cancer section in Genetics of Gastric Cancer.
When an individual meets HDGC genetic testing criteria but genetic testing does not reveal a CDH1pathogenic variant, CTNNA1 genetic testing is recommended.
Personal History Criteria
- Diffuse gastric cancer diagnosed at age 49 years or younger.
- Diffuse gastric cancer diagnosed at any age in individuals of Māori descent.
- Diffuse gastric cancer and cleft lip or cleft palate diagnosed in the same individual.
- Diffuse gastric cancer and a first-degree relative (FDR) with cleft lip or cleft palate.
- Diffuse gastric cancer and lobular breast cancer, both diagnosed in the same individual at age 69 years or younger.
- Bilateral lobular breast cancer diagnosed at age 69 years or younger.
- Gastric signet ring cell carcinoma in situ or pagetoid spread of gastric signet ring cells diagnosed at age 49 years or younger.
Family History Criteria a
- 2 or more cases of gastric cancer (with at least 1 diffuse gastric cancer case) occurring in the same family, regardless of age.
- 1 or more cases of diffuse gastric cancer diagnosed at any age, and 1 or more cases of lobular breast cancer diagnosed at age 69 years or younger (these cancers must occur in different family members).
- 2 or more cases of lobular breast cancer diagnosed in family members at age 49 years or younger.
a Family members must be FDRs or second-degree relatives of each other. The IGCLC recommends that affected family members undergo genetic testing, when possible. Providers may want to consider testing tissue (tumor tissue or healthy tissue) from deceased, affected relatives if a family does not have living individuals with breast or gastric cancers. If none of these options are feasible, providers can consider performing genetic testing in unaffected family members. Diffuse gastric cancer is generally diagnosed at an advanced stage (i.e., stage III or stage IV).[1]
References:
- Blair VR, McLeod M, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21 (8): e386-e397, 2020.
Definition and Management of Hereditary Diffuse Gastric Cancer (HDGC)–Like Families
CDH1germline pathogenic variants are found in 30% to 40% of families with clinically defined HDGC from different ethnic backgrounds.[1,2] The 2020 International Gastric Cancer Linkage Consortium defines families as HDGC-like if the following criteria are met: 1) a CDH1 or CTNNA1 pathogenic variant is not found, 2) there is at least one confirmed case of diffuse gastric cancer in the family, and 3) another gastric cancer or lobular breast cancer is found in first-degree or second-degree relatives.
In HDGC-like families, affected individuals and their FDRs may be considered for yearly endoscopic surveillance for at least 2 years after receiving a negative CDH1 genetic test result.[3] Surveillance begins at age 40 years or 10 years before the earliest case of gastric cancer in the family. HDGC surveillance can begin at, but not before, age 18 years. Surveillance intervals can be prolonged at the discretion of the endoscopist after the individual has participated in annual screening for 2 years. The decision to prolong screening intervals can be based on an individual's previous endoscopy findings and on his/her family history. Risk-reducing total gastrectomy is not advised when endoscopies are negative in HDGC-like families because the level of gastric cancer risk in these individuals is uncertain.
References:
- Lynch HT, Grady W, Suriano G, et al.: Gastric cancer: new genetic developments. J Surg Oncol 90 (3): 114-33; discussion 133, 2005.
- Suriano G, Oliveira C, Ferreira P, et al.: Identification of CDH1 germline missense mutations associated with functional inactivation of the E-cadherin protein in young gastric cancer probands. Hum Mol Genet 12 (5): 575-82, 2003.
- Blair VR, McLeod M, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21 (8): e386-e397, 2020.
Genetics
Hereditary diffuse gastric cancer (HDGC) was first identified in a large, indigenous Māori kindred from New Zealand. These individuals had a germline pathogenic variant in the CDH1gene. [1] Since this initial report, many studies have found that HDGC is a rare, autosomal dominant disorder that results in increased diffuse gastric cancer and lobular breast cancer risks.[1,2,3,4]
Molecular Biology
In 1995, the CDH1 (cadherin-1) gene was mapped to chromosome 16q22.1.[5] The CDH1 gene spans a region of approximately 100 kb, with 16 exons and a 65-kb-long intron 2.[5] The intron-exon boundaries are highly conserved. Intron 1 contains a 5′ high-density CpG island that may be implicated in transcriptional regulation during embryogenesis and cancer development.[5] The CDH1 gene encodes the E-cadherin transmembrane glycoprotein, which is expressed in epithelial tissue and is responsible for calcium-dependent cell–cell adhesion.[6] E-cadherin is the first adhesion molecule that is expressed at the 8-cell stage during embryonic development. This protein is essential for the compaction of the morula and subsequent organization of epithelial tissues. The E-cadherin protein is crucial for the establishment and maintenance of tissue architecture and homeostasis.[7,8] E-cadherin primarily forms adherens junctions via homophilic binding of the extracellular domain of cadherins that is presented on neighboring cells.[9] These adhesion structures act as tumor suppressors, preventing tumor invasion and metastasis.[6] The cytoplasmic domain of E-cadherin is connected to the actin cytoskeleton through various catenins (alpha, beta, and p120 catenins), providing the cell with structure, while also mediating cellular signaling.[10,11] E-cadherin regulates basic cellular processes like cell proliferation, migration, apoptosis, and invasion.[12] E-cadherin is crucial for maintaining tissue homeostasis, and in effect, E-cadherin loss can lead to pathogenic effects in cells.[13]
In 1998, it was determined that germline, heterozygous pathogenic variants in the CDH1 tumor suppressor gene can cause HDGC.[1] Most CDH1 pathogenic variants generate premature termination codons, resulting in loss of protein function due to nonsense-mediated decay.[14] The clinical significance of CDH1 sequence variants fall along a gradient, ranging from pathogenic to benign. CDH1 gene-specific interpretation guidelines exist to determine sequence variant pathogenicity based on the American College of Medical Genetics and Genomics and the Association for Molecular Pathology's (ACMG/AMP) classification framework.[15,16] According to the CDH1 gene-specific criteria, only missense variants that have been shown to affect splicing are classified as pathogenic or likely pathogenic variants. All other CDH1 missense variants are classified as variants of unknown significance (VUS). A retrospective review of clinical data (from European laboratories) that analyzed 854 CDH1 carriers supported this classification rule.[17] The study found that CDH1 pathogenic/likely pathogenic variants were positively associated with HDGC-related phenotypes. There was no evidence for a positive association between HDGC-related phenotypes and CDH1 missense VUS.
A systematic review of the literature from 1998 to 2021 identified 571 germline CDH1 pathogenic variants, with 387 (67.8%) variants reported in 108 different families.[18] The largest clusters of CDH1 pathogenic variants were identified in Central Europe, North America, Northern Europe, New Zealand (Māori), and South America. However, no studies in the review focused on populations from African and Middle Eastern countries. Only one study was included from an Asian country (Japan). A 2022 study of Chinese patients who met HDGC criteria identified two CDH1 pathogenic variants that were not previously reported.[19]
Genotype-Phenotype Correlations
CDH1 pathogenic variants demonstrate considerable heterogeneity with the types of cancers and the ages of cancer onset seen in carriers. However, a review of available CDH1 literature from 1985 to 2018 did not definitively establish genotype -phenotype correlations that could help direct patient management. This review found that CDH1 pathogenic variants are evenly distributed throughout the gene without a preferential variant type (i.e., nonsense, missense, etc.) or location.[13] An investigation of 152 families with HDGC analyzed the associations between an individual's CDH1 germline pathogenic variant status and clinical HDGC phenotype. This study found that variants in the intracellular E-cadherin region of the CDH1 gene seemed to protect individuals from developing cancer at young ages (odds ratio [OR], 0.2; P = .0071). Similarly, variants in the linker regions of the CDH1 gene were protective against breast cancer (OR, 0.35; P = .0493).[20] Another study analyzed the frequency of different CDH1 pathogenic variant subtypes in asymptomatic individuals (n = 289) and individuals diagnosed with gastric cancer (n = 224) from HDGC families.[21] In CDH1 carriers, splicing variants were found in 30.4% of healthy individuals and in 15.2% of patients with gastric cancer (P = .0076). Missense variants were found more often in healthy subjects (22.2%) than in individuals with gastric cancer (18.3%), but this difference did not reach statistical significance. The authors of this study concluded that not all CDH1 germline pathogenic variants conferred the same gastric cancer risk.
Possible CDH1 genotype-phenotype correlations have been observed in families with HDGC and cleft lip or cleft palate. However, it is unclear which CDH1 pathogenic variants lead to cleft lip or cleft palate. In 2006, CDH1 splice site variants were found in two families with HDGC and cleft lip or cleft palate. The splice site variants resulted in complex, aberrant splicing in lymphocytes.[22] However, CDH1 splice site variants were subsequently identified in HDGC families without cleft lip or cleft palate.[13,23] A review article noted that families with a combined phenotype of HDGC and cleft lip or cleft palate had CDH1 variants within extracellular domains. However, CDH1 variants within extracellular domains have also been seen in families with isolated HDGC.[24] A systematic review of 280 CDH1 pathogenic variants showed that variants found in individuals with cleft lip or cleft palate seemed to cluster closely in similar regions of the CDH1 gene. These variants often resided within linker regions between extracellular domains.[25] The differences between pathogenic variants in individuals with HDGC plus cleft lip or cleft palate versus those in individuals with HDGC who did not have cleft lip or cleft palate were not robust enough to reliably predict the phenotype for a given CDH1 variant. However, these differences may help providers more accurately assess cleft lip or cleft palate risk in individuals with specific CDH1 variants.
The term, hereditary lobular breast cancer, was created by the International Gastric Cancer Linkage Consortium to describe individuals with a CDH1 pathogenic variant and personal/family histories of lobular breast cancer, but no known personal/family histories of gastric cancer.[26] Given the lack of data supporting a genotype-phenotype distinction between hereditary lobular breast cancer and HDGC, a study assessed the prevalence of occult gastric cancer in patients with hereditary lobular breast cancer and a CDH1 pathogenic variant.[27] The results indicated that germline CDH1 pathogenic variants were not associated with mutually exclusive hereditary lobular breast cancer or HDGC phenotypes. Furthermore, the rates of occult gastric cancer were the same for patients with hereditary lobular breast cancer and for patients with HDGC. The authors emphasized that patients who had previously been given a hereditary lobular breast cancer diagnosis should undergo gastric cancer surveillance and receive counseling regarding risk-reducing gastrectomy.
Overall, there are insufficient data regarding possible CDH1 genotype-phenotype correlations. These findings are not substantial enough to guide individualized CDH1 cancer risk assessment and management.
References:
- Guilford P, Hopkins J, Harraway J, et al.: E-cadherin germline mutations in familial gastric cancer. Nature 392 (6674): 402-5, 1998.
- Hansford S, Kaurah P, Li-Chang H, et al.: Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 1 (1): 23-32, 2015.
- Brooks-Wilson AR, Kaurah P, Suriano G, et al.: Germline E-cadherin mutations in hereditary diffuse gastric cancer: assessment of 42 new families and review of genetic screening criteria. J Med Genet 41 (7): 508-17, 2004.
- Kaurah P, MacMillan A, Boyd N, et al.: Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 297 (21): 2360-72, 2007.
- Berx G, Staes K, van Hengel J, et al.: Cloning and characterization of the human invasion suppressor gene E-cadherin (CDH1). Genomics 26 (2): 281-9, 1995.
- Yelskaya Z, Bacares R, Salo-Mullen E, et al.: CDH1 Missense Variant c.1679C>G (p.T560R) Completely Disrupts Normal Splicing through Creation of a Novel 5' Splice Site. PLoS One 11 (11): e0165654, 2016.
- Paredes J, Figueiredo J, Albergaria A, et al.: Epithelial E- and P-cadherins: role and clinical significance in cancer. Biochim Biophys Acta 1826 (2): 297-311, 2012.
- van Roy F, Berx G: The cell-cell adhesion molecule E-cadherin. Cell Mol Life Sci 65 (23): 3756-88, 2008.
- Shapiro L, Fannon AM, Kwong PD, et al.: Structural basis of cell-cell adhesion by cadherins. Nature 374 (6520): 327-37, 1995.
- Aberle H, Schwartz H, Kemler R: Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem 61 (4): 514-23, 1996.
- Okamoto R, Irie K, Yamada A, et al.: Recruitment of E-cadherin associated with alpha- and beta-catenins and p120ctn to the nectin-based cell-cell adhesion sites by the action of 12-O-tetradecanoylphorbol-13-acetate in MDCK cells. Genes Cells 10 (5): 435-45, 2005.
- Kim NG, Koh E, Chen X, et al.: E-cadherin mediates contact inhibition of proliferation through Hippo signaling-pathway components. Proc Natl Acad Sci U S A 108 (29): 11930-5, 2011.
- Figueiredo J, Melo S, Carneiro P, et al.: Clinical spectrum and pleiotropic nature of CDH1 germline mutations. J Med Genet 56 (4): 199-208, 2019.
- Karam R, Carvalho J, Bruno I, et al.: The NMD mRNA surveillance pathway downregulates aberrant E-cadherin transcripts in gastric cancer cells and in CDH1 mutation carriers. Oncogene 27 (30): 4255-60, 2008.
- Luo X, Maciaszek JL, Thompson BA, et al.: Optimising clinical care through CDH1-specific germline variant curation: improvement of clinical assertions and updated curation guidelines. J Med Genet 60 (6): 568-575, 2023.
- Richards S, Aziz N, Bale S, et al.: Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17 (5): 405-24, 2015.
- Garcia-Pelaez J, Barbosa-Matos R, Lobo S, et al.: Genotype-first approach to identify associations between CDH1 germline variants and cancer phenotypes: a multicentre study by the European Reference Network on Genetic Tumour Risk Syndromes. Lancet Oncol 24 (1): 91-106, 2023.
- Corso G, Tagliaferri V, Massari G, et al.: CDH1 mutations recurrence and global clustering in genetically tested families with hereditary diffuse gastric cancer syndrome: results from a systematic study. Fam Cancer 22 (2): 187-192, 2023.
- Pan Z, Fu Z, Luo C, et al.: CDH1 germline mutations in a Chinese cohort with hereditary diffuse gastric cancer. J Cancer Res Clin Oncol 148 (8): 2145-2151, 2022.
- Lo W, Zhu B, Sabesan A, et al.: Associations of CDH1 germline variant location and cancer phenotype in families with hereditary diffuse gastric cancer (HDGC). J Med Genet 56 (6): 370-379, 2019.
- Corso G, Magnoni F, Massari G, et al.: CDH1 germline mutations in healthy individuals from families with the hereditary diffuse gastric cancer syndrome. J Med Genet 59 (4): 313-317, 2022.
- Frebourg T, Oliveira C, Hochain P, et al.: Cleft lip/palate and CDH1/E-cadherin mutations in families with hereditary diffuse gastric cancer. J Med Genet 43 (2): 138-42, 2006.
- Corso G, Marrelli D, Pascale V, et al.: Frequency of CDH1 germline mutations in gastric carcinoma coming from high- and low-risk areas: metanalysis and systematic review of the literature. BMC Cancer 12: 8, 2012.
- Obermair F, Rammer M, Burghofer J, et al.: Cleft lip/palate and hereditary diffuse gastric cancer: report of a family harboring a CDH1 c.687 + 1G > A germline mutation and review of the literature. Fam Cancer 18 (2): 253-260, 2019.
- Selvanathan A, Nixon CY, Zhu Y, et al.: CDH1 Mutation Distribution and Type Suggests Genetic Differences between the Etiology of Orofacial Clefting and Gastric Cancer. Genes (Basel) 11 (4): , 2020.
- Blair VR, McLeod M, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21 (8): e386-e397, 2020.
- Gamble LA, Rossi A, Fasaye GA, et al.: Association Between Hereditary Lobular Breast Cancer Due to CDH1 Variants and Gastric Cancer Risk. JAMA Surg 157 (1): 18-22, 2022.
Clinical Manifestations
Gastric Cancer Risk
In CDH1pathogenic variant carriers, lifetime risk of diffuse gastric cancer by age 80 years ranges from 37% to 70% in men and 25% to 83% in women.[1,2,3,4] The average age of gastric cancer onset ranges from 38 to 80 years, with the earliest cancer reported at age 14 years.[1,2,3,5,6] It is important to recognize that the diffuse gastric cancer in CDH1 pathogenic variant carriers often begins as multifocal, microscopic deposits of signet ring cell carcinoma. Hereditary diffuse gastric cancer (HDGC) arises in the neck region of the gastric glands (rather than on the surface epithelium), where dysplasia from environmental and inflammatory risk factors tends to form. These signet ring cell carcinomas grow diffusely, but subtly, into the adjoining gastric mucosa and submucosa. CDH1-associated diffuse gastric cancer has a similar presentation and clinical course as sporadic diffuse gastric cancer. Minimally invasive therapy is an option for precursor lesions identified on endoscopy, but most enlarging signet ring cell lesions associated with HDGC cannot be detected until they reach an advanced stage. HDGC begins as multifocal signet ring cells that are found throughout the stomach. It is unclear if this also occurs in sporadic diffuse gastric cancer.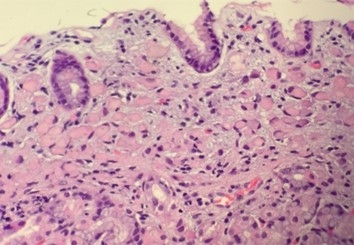
Figure 1. High-power image of a gastric biopsy tissue sample showing signet ring cell carcinoma. Note the absence of gland formation, which indicates that this lesion is poorly-differentiated carcinoma. Also note the overlying epithelium, which although effaced by the presence of underlying tumor, is otherwise normal.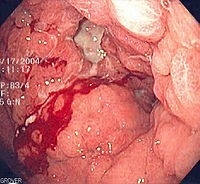
Figure 2. Endoscopic image of diffuse gastric cancer with typical linitis plastica involvement. This occurs at an advanced stage. The gastric mucosa and submucosa are diffusely infiltrated such that the entire stomach becomes stiff, losing contractility and the ability to digest food. This leads to the typical symptoms of nausea and vomiting. Biopsy is generally informative, but a very superficial biopsy may be negative for cancer cells. Hence, it is imperative for pathologists to carefully search biopsy specimens for isolated signet ring cells.
Breast Cancer Risk
In the general population, most breast cancers are ductal, while about 10% are lobular.[7] In contrast, most breast cancers in women with CDH1germline pathogenic variants are lobular.[8,9,10]Penetrance estimates are 39% to 55% for invasive lobular breast cancer (ILC) in women with CDH1 pathogenic variants to age 80 years.[1,2,3,11] Studies have shown that ILC risk begins to increase before age 30 years, with an average age of onset between 46 and 50 years.[2,4] The histopathological and immunohistochemical features of germline CDH1-related ILC and sporadic ILC are indistinguishable.[9] Overall, ILC tends to be multicentric, and tumors do not form well-defined masses. Instead, ILCs are composed of small, infiltrating epithelial cells, which are dispersed individually in a single-file, linear pattern in a fibrous stroma. ILCs lose expression of the cell–cell adhesion molecule, E-cadherin.[12] ILC is associated with an advanced stage at presentation, and 10% to 15% of cases are bilateral.[12,13] ILC features are often associated with a good prognosis because they are usually low grade, estrogen receptor (ER)–positive, and human epidermal growth factor receptor two (HER2)–negative.[14] However, ILC tends to have a higher risk of distant recurrence after 10 years when compared with ductal breast cancer.[13] ILC also tends to metastasize to unusual sites, like the gastrointestinal tract and the meninges.[15] Guidelines for ILC treatment in CDH1 carriers do not differ from treatment guidelines for those with sporadic ILCs.[16]
References:
- Pharoah PD, Guilford P, Caldas C, et al.: Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 121 (6): 1348-53, 2001.
- Roberts ME, Ranola JMO, Marshall ML, et al.: Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol 5 (9): 1325-1331, 2019.
- Xicola RM, Li S, Rodriguez N, et al.: Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet 56 (12): 838-843, 2019.
- Hansford S, Kaurah P, Li-Chang H, et al.: Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 1 (1): 23-32, 2015.
- Delgado E, León-Ponte M, Villahermosa ML, et al.: Analysis of HIV type 1 protease and reverse transcriptase sequences from Venezuela for drug resistance-associated mutations and subtype classification: a UNAIDS study. AIDS Res Hum Retroviruses 17 (8): 753-8, 2001.
- Caldas C, Carneiro F, Lynch HT, et al.: Familial gastric cancer: overview and guidelines for management. J Med Genet 36 (12): 873-80, 1999.
- Li CI, Anderson BO, Daling JR, et al.: Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA 289 (11): 1421-4, 2003.
- Corso G, Intra M, Trentin C, et al.: CDH1 germline mutations and hereditary lobular breast cancer. Fam Cancer 15 (2): 215-9, 2016.
- Corso G, Figueiredo J, La Vecchia C, et al.: Hereditary lobular breast cancer with an emphasis on E-cadherin genetic defect. J Med Genet 55 (7): 431-441, 2018.
- Masciari S, Larsson N, Senz J, et al.: Germline E-cadherin mutations in familial lobular breast cancer. J Med Genet 44 (11): 726-31, 2007.
- Kaurah P, MacMillan A, Boyd N, et al.: Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 297 (21): 2360-72, 2007.
- Mouabbi JA, Hassan A, Lim B, et al.: Invasive lobular carcinoma: an understudied emergent subtype of breast cancer. Breast Cancer Res Treat 193 (2): 253-264, 2022.
- Pilonis ND, Tischkowitz M, Fitzgerald RC, et al.: Hereditary Diffuse Gastric Cancer: Approaches to Screening, Surveillance, and Treatment. Annu Rev Med 72: 263-280, 2021.
- Rakha EA, El-Sayed ME, Powe DG, et al.: Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. Eur J Cancer 44 (1): 73-83, 2008.
- Lamovec J, Bracko M: Metastatic pattern of infiltrating lobular carcinoma of the breast: an autopsy study. J Surg Oncol 48 (1): 28-33, 1991.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4.2023. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed October 25, 2024.
Management
Surgical Intervention: Risk-Reducing Gastrectomy
Risk-reducing total gastrectomy (RRTG) with esophagojejunostomy remains the treatment of choice for individuals with CDH1pathogenic variants.[1] The RRTG specimens from individuals with CDH1 pathogenic variants often consist of microscopic foci of intramucosal signet ring cell carcinoma (SRCC), usually confined to the superficial lamina propria, without infiltration beneath the muscularis.[2,3,4,5,6] Intramucosal signet ring cells in the lamina propria may be classified as invasive, but they follow an indolent course. Microscopic foci of SRCC may never grow into advanced diffuse gastric cancer because early-stage CDH1-associated lesions are typically transient, with an indolent immunoexpression phenotype.[7] A systematic review of 224 reported RRTGs (performed in individuals with CDH1 pathogenic variants) found gastric cancer in 85.4% of specimens.[8] Of the 224 specimens, histopathology findings were available for 219. In 32 (14.6%) cases, cancer was not detected. In 159 (72.6%) cases, at least one focus of SRCC was identified. In 28 (12.8%) cases, an invasive tumor was identified. Overall, 33% of individuals without family histories of gastric cancer had cancer detected in their gastrectomy specimens, while 85% of patients with family histories of gastric cancer had cancer detected in their gastrectomy specimens. A retrospective review of 101 RRTG specimens (from asymptomatic patients with CDH1 pathogenic variants) identified early-stage pT1a SRCC in 85% of patients.[2] Younger (aged <18 y) or older (aged >70 y) individuals require a more individualized approach, given the high morbidity rates associated with this surgery. Gastrectomy can be performed robotically, laparoscopically, or in a traditional, open fashion.[9] Care must always be taken to ensure that surgical margins are free of gastric epithelium.
Functional outcomes after RRTG vary, but weight loss is universal. One series reported an average weight loss of 15% to 20% postsurgery.[2,3,4] Most patients eventually establish a new normal after surgery, but their nutritional statuses must be closely monitored. Other risks after gastrectomy include the following: nutritional deficiencies, anastomotic leaks, intra-abdominal infections, gastrointestinal symptoms like diarrhea and reflux, and a mortality rate that ranges from 3% to 6%.[10] A review of 54 patients who had a RRTG (median age, 41 y) showed that median quality of life was very good after 4.6 years of follow-up.[4]
An international group of experts have published consensus guidelines for follow-up of patients with hereditary diffuse gastric cancer (HDGC) after RRTG.[11] They recommend regular follow-up with a multidisciplinary team who will review the patient's diet, weight, and symptoms. Laboratory tests are also recommended to document general health, nutrition, bone health, and cancer risk (i.e., breast cancer). The guidelines state that vitamin and mineral supplementation are to be considered. Although most of the statements in the guidelines were supported by low levels of evidence, they provide a framework for management of patients with HDGC after RRTG.
Endoscopic Surveillance
RRTG is generally considered the treatment of choice for patients with HDGC since it minimizes gastric cancer risk by removing high-risk gastric tissue (given the lack of consistently identifiable precursor lesions on endoscopy).[1] When gastrectomy is not performed, endoscopic surveillance is an alternate option. When gastrectomy is performed, multiple microscopic foci of SRCC are typically found. In contrast, targeted, random sampling of the stomach during endoscopy often fails to identify microscopic foci of SRCC. Since diffuse gastric cancer often results in a grim prognosis and is usually found at an advanced stage, most experts recommend total gastrectomy, regardless of the number of signet ring cell lesions found during endoscopic sampling. In one series, six patients with CDH1 pathogenic variants from one family (mean age, 54 y) underwent RRTG despite an absence of symptoms and normal esophagogastroduodenoscopies (EGDs) with negative random biopsies of the stomach. Microscopic foci of SRCC were present in all individuals who underwent RRTG.[12] Researchers performed surveillance EGDs on 20 CDH1 carriers.[13] Suspicious lesions were not found in any participants during EGD examinations. However, SSRC was found in 40% of endoscopic biopsy samples. Thirty or more random endoscopic biopsies were performed in each participant.
Although RRTG remains the gold standard recommendation for HDGC, a multidisciplinary discussion on management options is favored based on the following: emerging evidence on endoscopic surveillance, the unpredictable rate of progression from intramucosal signet cells and T1a lesions to advanced gastric cancer, and morbidity of surgery. There is a role for endoscopic surveillance in HDGC, specifically for patients who decline surgery or choose to defer RRTG when they are diagnosed with HDGC. A prospective cohort study analyzed 270 asymptomatic CDH1 carriers (median age, 46.6 y; interquartile range [IQR], 36.5–59.8) who declined RRTG and participated in endoscopic surveillance with the Cambridge protocol (9%) or Bethesda protocol (91%).[5] These individuals underwent surveillance for a median period of 31.1 months (IQR, 17.1–42.1) at recommended 6-month to 12-month intervals. Surveillance identified SSRC in 63% (76/120) of patients. Ninety-seven percent (74/76) of patients with visibly normal mucosa had malignancies detected on random biopsies. In 93% of patients who had RRTG, multifocal stage IA gastric carcinoma was detected, with no stage III or IV cancers detected. The authors concluded that endoscopic surveillance is an acceptable surveillance strategy in those who decline RRTG.
Other scenarios that may prompt endoscopic surveillance include the following:[1]
- Patients with family histories suggestive of HDGC but for whom no pathogenic variant has been identified.
- Patients with family histories of intestinal-type gastric cancer. This type of gastric cancer is usually not associated with CDH1 pathogenic variants. Therefore, endoscopic surveillance needs to be individualized for these patients.
- Patients who have a CDH1 pathogenic variant and choose to defer RRTG for a year or more. This includes patients who undergo genetic testing at a young age and test positive for a CDH1 pathogenic variant. Risk-reducing gastrectomy is generally not recommended in individuals who are younger than 18 to 20 years. Despite being warned about endoscopic biopsy's poor rate of HDGC precursor detection, many patients (especially those who are older) will only consider gastrectomy in the event of a positive biopsy.
There are no definitive, identifiable features of early diffuse gastric cancer or diffuse gastric cancer precursors that can be found on endoscopy. Targeted biopsies of ulcers, erythema, nodularity/nodules, polyps, erosions, and scars revealed SSRC in less than 10% of samples.[5] Pale spots had the highest rate of SSRC detected. Hence, a liberal number of random biopsies are taken from the gastric mucosa during endoscopy since the epithelium that covers SSRC generally appears normal.[5,14] Random biopsies enhanced detection of SSRC more than targeted biopsies did. Reports suggested that individuals with HDGC can have many microscopic tumors in the body-antrum transitional zone.[6] However, a more recent study suggested that these tumors typically involve the oxyntic mucosa and the proximal stomach instead.[15]
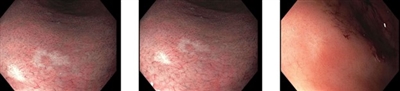
Figure 3. Several small, white, slightly depressed areas are best seen with narrow-band imaging. These lesions can resemble scars from previous endoscopic biopsies. However, the images above are from a patient who did not have a prior esophagogastroduodenoscopy. A microscopic signet ring cell carcinoma focus was found on this biopsy, although nontargeted biopsies in this patient were also positive for signet ring cell carcinoma.
Another study reported on 33 patients with CDH1 pathogenic variants (median age, 32 y) who were members of the original Māori family in which HDGC was first discovered. Participants underwent 99 surveillance endoscopies, 93 of which were performed with a mucolytic N-acetylcysteine followed by Congo red–methylene blue dye.[16] Twenty-four of 93 endoscopies exhibited one or more flat, pale patches, which were between 2 mm and 10 mm in diameter. SRCC was found in 23 of the 56 patches that were biopsied (41%). The authors concluded that these patches could be better characterized with Congo red–methylene blue chromogenic dyes. Biopsy yields increased when these dyes were used. The authors did, however, express concern that Congo red dye may have carcinogenic properties, and its use has not persisted.
If an endoscopic approach is taken for HDGC management, endoscopic surveillance is recommended annually unless signet ring cell features are found. If signet ring cell lesions are found on endoscopy, discussion with a multidisciplinary team is warranted.[1] However, diffuse gastric cancer risk remains unclear in individuals with foci of signet ring cells found on endoscopy, since the natural course of these foci are unknown.
Breast Cancer Risk Management
In females with a CDH1 pathogenic variant, screening modalities for breast cancer include the following:
Breast magnetic resonance imaging (MRI):
- The National Comprehensive Cancer Network (NCCN) recommends consideration of breast MRI with contrast beginning at age 30 years.[17]
- The International Gastric Cancer Linkage Consortium (IGCLC) recommends that annual breast MRI begin at age 30 years since mammography is not effective at identifying lobular breast cancer.[1,18]
Mammography:
- NCCN and IGCLC have differing recommendations regarding the age to begin mammography screening.
- NCCN recommends that annual mammography screenings begin at age 30 years.[17]
- IGCLC recommends that annual mammography screenings begin between the ages of 35 and 40 years, based on the patient's breast cancer risk factors, like breast density.[1]
Other imaging:
- The IGCLC states that supplementary screening of dense breasts with ultrasound can be considered, especially when MRI is not available, contraindicated, or declined.[1]
Bilateral risk-reducing mastectomy can be considered for women with a CDH1 pathogenic variant based on a strong family history of breast cancer, additional breast cancer risk factors, and/or personal choice.[1,17]
References:
- Blair VR, McLeod M, Carneiro F, et al.: Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 21 (8): e386-e397, 2020.
- Vos EL, Salo-Mullen EE, Tang LH, et al.: Indications for Total Gastrectomy in CDH1 Mutation Carriers and Outcomes of Risk-Reducing Minimally Invasive and Open Gastrectomies. JAMA Surg 155 (11): 1050-1057, 2020.
- Strong VE, Gholami S, Shah MA, et al.: Total Gastrectomy for Hereditary Diffuse Gastric Cancer at a Single Center: Postsurgical Outcomes in 41 Patients. Ann Surg 266 (6): 1006-1012, 2017.
- Stillman MD, Kusche N, Toledano S, et al.: Short and long-term outcomes of prophylactic total gastrectomy in 54 consecutive individuals with germline pathogenic mutations in the CDH1 gene. J Surg Oncol 126 (8): 1413-1422, 2022.
- Asif B, Sarvestani AL, Gamble LA, et al.: Cancer surveillance as an alternative to prophylactic total gastrectomy in hereditary diffuse gastric cancer: a prospective cohort study. Lancet Oncol 24 (4): 383-391, 2023.
- Charlton A, Blair V, Shaw D, et al.: Hereditary diffuse gastric cancer: predominance of multiple foci of signet ring cell carcinoma in distal stomach and transitional zone. Gut 53 (6): 814-20, 2004.
- van der Post RS, Gullo I, Oliveira C, et al.: Histopathological, Molecular, and Genetic Profile of Hereditary Diffuse Gastric Cancer: Current Knowledge and Challenges for the Future. Adv Exp Med Biol 908: 371-91, 2016.
- Corso G, Magnoni F, Nicastro V, et al.: Global distribution of prophylactic total gastrectomy in E-cadherin (CDH1) mutations. Semin Oncol 49 (2): 130-135, 2022.
- Bres C, Voron T, Benhaim L, et al.: Management of Pathogenic CDH1 Variant Carriers Within the FREGAT Network: A Multicentric Retrospective Study. Ann Surg 276 (5): 830-837, 2022.
- Davis JL, Ripley RT: Postgastrectomy Syndromes and Nutritional Considerations Following Gastric Surgery. Surg Clin North Am 97 (2): 277-293, 2017.
- Roberts G, Benusiglio PR, Bisseling T, et al.: International Delphi consensus guidelines for follow-up after prophylactic total gastrectomy: the Life after Prophylactic Total Gastrectomy (LAP-TG) study. Gastric Cancer 25 (6): 1094-1104, 2022.
- Norton JA, Ham CM, Van Dam J, et al.: CDH1 truncating mutations in the E-cadherin gene: an indication for total gastrectomy to treat hereditary diffuse gastric cancer. Ann Surg 245 (6): 873-9, 2007.
- Jacobs MF, Dust H, Koeppe E, et al.: Outcomes of Endoscopic Surveillance in Individuals With Genetic Predisposition to Hereditary Diffuse Gastric Cancer. Gastroenterology 157 (1): 87-96, 2019.
- Lee CYC, Olivier A, Honing J, et al.: Endoscopic surveillance with systematic random biopsy for the early diagnosis of hereditary diffuse gastric cancer: a prospective 16-year longitudinal cohort study. Lancet Oncol 24 (1): 107-116, 2023.
- Fujita H, Lennerz JK, Chung DC, et al.: Endoscopic surveillance of patients with hereditary diffuse gastric cancer: biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am J Surg Pathol 36 (11): 1709-17, 2012.
- Shaw D, Blair V, Framp A, et al.: Chromoendoscopic surveillance in hereditary diffuse gastric cancer: an alternative to prophylactic gastrectomy? Gut 54 (4): 461-8, 2005.
- National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic. Version 2.2024. Plymouth Meeting, Pa: National Comprehensive Cancer Network, 2023. Available online with free registration. Last accessed September 18, 2024.
- Pereslucha AM, Wenger DM, Morris MF, et al.: Invasive Lobular Carcinoma: A Review of Imaging Modalities with Special Focus on Pathology Concordance. Healthcare (Basel) 11 (5): , 2023.
Latest Updates to This Summary (10 / 25 / 2024)
The PDQ cancer information summaries are reviewed regularly and updated as new information becomes available. This section describes the latest changes made to this summary as of the date above.
Added text to state that in patients with CDH1 pathogenic variants, early stage gastric cancer is characterized by intramucosal foci of signet ring cell carcinoma while advanced gastric cancer presents as poorly cohesive diffuse type carcinoma with very few typical signet ring cells (cited van der Post et al. as reference 1).
Revised text to state that the first study to provide CTNNA1 penetrance estimates reported up to a 57% lifetime risk of developing gastric cancer in families from European hereditary cancer registries (cited Coudert et al. as reference 12).
Genetic Testing Criteria for Hereditary Diffuse Gastric Cancer
Revised text to state that when two or more different cancer types are involved, at least one must be diffuse gastric cancer with signet ring cell carcinoma or lobular breast cancer.
Definition and Management of Hereditary Diffuse Gastric Cancer (HDGC)–Like Families
Revised text to state that the 2020 International Gastric Cancer Linkage Consortium defines families as HDGC-like if the following criteria are met: 1) a CDH1 or CTNNA1 pathogenic variant is not found, 2) there is at least one confirmed case of diffuse gastric cancer in the family, and 3) another gastric cancer or lobular breast cancer is found in first-degree or second-degree relatives.
The Molecular Biology subsection was extensively revised.
The Surgical Intervention: Risk-Reducing Gastrectomy subsection was extensively revised.
The Endoscopic Surveillance subsection was extensively revised.
This summary is written and maintained by the PDQ Cancer Genetics Editorial Board, which is editorially independent of NCI. The summary reflects an independent review of the literature and does not represent a policy statement of NCI or NIH. More information about summary policies and the role of the PDQ Editorial Boards in maintaining the PDQ summaries can be found on the About This PDQ Summary and PDQ® Cancer Information for Health Professionals pages.
About This PDQ Summary
Purpose of This Summary
This PDQ cancer information summary for health professionals provides comprehensive, peer-reviewed, evidence-based information about the genetics of hereditary diffuse gastric cancer. It is intended as a resource to inform and assist clinicians in the care of their patients. It does not provide formal guidelines or recommendations for making health care decisions.
Reviewers and Updates
This summary is reviewed regularly and updated as necessary by the PDQ Cancer Genetics Editorial Board, which is editorially independent of the National Cancer Institute (NCI). The summary reflects an independent review of the literature and does not represent a policy statement of NCI or the National Institutes of Health (NIH).
Board members review recently published articles each month to determine whether an article should:
- be discussed at a meeting,
- be cited with text, or
- replace or update an existing article that is already cited.
Changes to the summaries are made through a consensus process in which Board members evaluate the strength of the evidence in the published articles and determine how the article should be included in the summary.
The lead reviewers for Hereditary Diffuse Gastric Cancer are:
- Doreen Agnese, MD (The Ohio State University)
- Ilana Cass, MD (Dartmouth-Hitchcock Medical Center)
- Lee-may Chen, MD (UCSF Helen Diller Family Comprehensive Cancer Center)
- Mary B. Daly, MD, PhD (Fox Chase Cancer Center)
- Grace-Ann O. Fasaye, ScM, CGC (National Cancer Institute)
- Gautam Mankaney, MD (Virginia Mason Franciscan Health)
- Tuya Pal, MD, FACMG, FCCMG (Vanderbilt-Ingram Cancer Center)
- Padma Sheila Rajagopal, MD, MPH, MSC (National Cancer Institute)
Any comments or questions about the summary content should be submitted to Cancer.gov through the NCI website's Email Us. Do not contact the individual Board Members with questions or comments about the summaries. Board members will not respond to individual inquiries.
Levels of Evidence
Some of the reference citations in this summary are accompanied by a level-of-evidence designation. These designations are intended to help readers assess the strength of the evidence supporting the use of specific interventions or approaches. The PDQ Cancer Genetics Editorial Board uses a formal evidence ranking system in developing its level-of-evidence designations.
Permission to Use This Summary
PDQ is a registered trademark. Although the content of PDQ documents can be used freely as text, it cannot be identified as an NCI PDQ cancer information summary unless it is presented in its entirety and is regularly updated. However, an author would be permitted to write a sentence such as "NCI's PDQ cancer information summary about breast cancer prevention states the risks succinctly: [include excerpt from the summary]."
The preferred citation for this PDQ summary is:
PDQ® Cancer Genetics Editorial Board. PDQ Hereditary Diffuse Gastric Cancer. Bethesda, MD: National Cancer Institute. Updated <MM/DD/YYYY>. Available at: https://www.cancer.gov/publications/pdq/information-summaries/genetics/hereditary-diffuse-gastric-cancer-hp-pdq. Accessed <MM/DD/YYYY>. [PMID: 37669414]
Images in this summary are used with permission of the author(s), artist, and/or publisher for use within the PDQ summaries only. Permission to use images outside the context of PDQ information must be obtained from the owner(s) and cannot be granted by the National Cancer Institute. Information about using the illustrations in this summary, along with many other cancer-related images, is available in Visuals Online, a collection of over 2,000 scientific images.
Disclaimer
The information in these summaries should not be used as a basis for insurance reimbursement determinations. More information on insurance coverage is available on Cancer.gov on the Managing Cancer Care page.
Contact Us
More information about contacting us or receiving help with the Cancer.gov website can be found on our Contact Us for Help page. Questions can also be submitted to Cancer.gov through the website's Email Us.
Last Revised: 2024-10-25
This information does not replace the advice of a doctor. Ignite Healthwise, LLC disclaims any warranty or liability for your use of this information. Your use of this information means that you agree to the Terms of Use and Privacy Policy. Learn how we develop our content.
Healthwise, Healthwise for every health decision, and the Healthwise logo are trademarks of Ignite Healthwise, LLC.


